
Pregnancy Unknown Location
Early IUP
Failed IUP
Early Non Viable pregnancy
Ectopic
Serial HCG
Copyright 2010
Definition
Spontaneous abortion is the expulsion of a non viable embryo or fetus usually caused by to chromosomal aberrations or environmental factors, occurring before the 20the week, though most occur before 12 weeks.
Structural changes that can lead to spontaneous abortion include uterine septae and anatomic anomalies like bicornuate uterus. Spontaneous abortions in the first trimester usually occur due to chromosomal anomalies of the fetus.
However, functional changes due to maternal infection or toxin exposure can lead to spontaneous abortion in the second trimester.
There are 4 clinical entities including threatened, inevitable, incomplete and complete spontaneous abortion.
Clinically threatened abortion is diagnosed and characterized by some bleeding and sometimes accompanied by pain in the first trimester. About half of these patients will recover and proceed to full term pregnancies.
When the symptoms are accompanied by a dilated cervix then abortion becomes “inevitable”. Bleeding and cramps are usually more prominent with inevitable abortion. The presence of products of conception in the discharged blood warrants examination by ultrasound. If products of conception are identified in the endometrial cavity the diagnosis is compatible with incomplete abortion and their absence confirms the diagnosis of a complete spontaneous abortion. Pain and bleeding subside when the abortion is complete.
Treatment depends on the stage of the the spontaneous abortion. A complete abortion requires no further treatment. An incomplete or inevitable abortion before 13 weeks is treated with suction dilatation and curettage though medical therapy (misorostol) can be used.
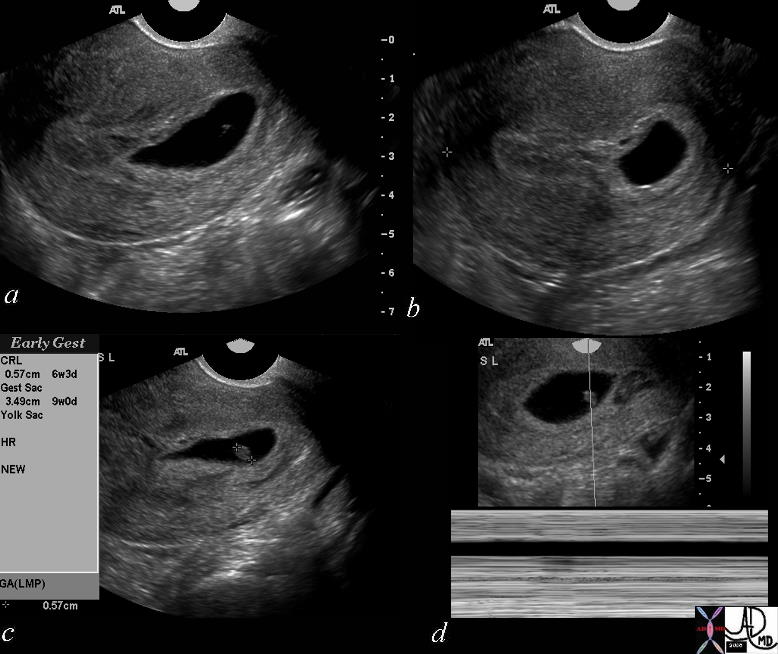
Gestational Sac Bulging into the Cervix |
|
The history is one of a young female presenting with vaginal bleeding with previous positive pregnancy test. The images show a deformed gestational sac bulging into the cervix. By ultrasound the gestational sac measured 3.49 cms consistent with GA of 9 weeks and CRL measures .49cms consistent with a gestational age of 6weeks and 3 days. No fetal heart was identified. An evolving spontaneous abortion was diagnosed uterus Courtesy Ashley Davidoff MD Copyright 2010 46592c01 |
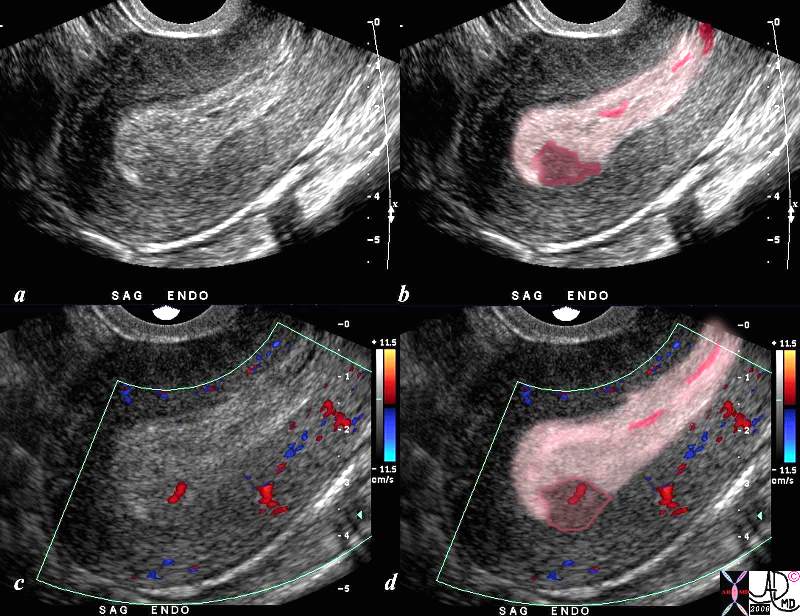
Retained Products of Conception |
| The ultrasound is from a 46 patient who presents with bleeding and pain 9 weeks into her pregnancy. Doppler study shows no fetal pole nor fetal heart beat. Findings include a thickened endometrium (light pink) with a focal area in the posterior and fundal portion of the endometrium (dark pink with red blood vessel inside) that enhances, consistent with retained products of conception and spontaneous abortion.
SAB US ultrasound USscan Courtesy Ashley Davidoff MD copyright |
The Common Vein Copyright 2010
Derfinition
Placental Abruption is a disorder of placental bleeding during pregnancy.
The cause is premature separation of the placenta from the uterine wall resulting in hemorrhage.
The structural change is characterized by partial or complete separation of the placenta from the uterine wall.
The functional changes are characterized by bleeding from the placenta and exposed endometrium. The blood may be confined to the uterine cavity or bleed through the cervical os. The functional changes may include insufficient blood flow to the fetus.
Women present clinically with bleeding accompanied by abdominal pain, uterine cramping and fetal distress.
Diagnosis is made based on clinical history and exam. Imaging with ultrasound is used to rule out placenta previa.
Treatment of severe bleeds is cesarean delivery. Vaginal delivery may be attempted in women with controlled bleeds and without fetal distress.
The Common Vein Copyright 2010
Definition
Placenta previa is a disorder of placental bleeding during pregnancy.
The cause is implantation of the placenta near or overlying the cervical os.
The result is bleeding in late pregnancy.
The structural change is characterized by placental implantation near the cervical os. As the placenta grows, part of the placenta may grow over the cervical os.
The functional changes are characterized by small disruptions in the attachment of the placenta to the endometrium causing placental bleeding through the cervical os.
Women most often present clinically with painless bleeding late in pregnancy. The amount of bleeding varies from small losses to severe hemorrhage.
Diagnosis is made with ultrasound imaging. Treatment depends on the amount of bleeding.
Small amounts of bleeding may be managed expectantly with steroids to promote fetal lung maturity and bed rest. Large bleeds are treated with immediate cesarean delivery.
Copyright 2010
Definition
Hydatidiform mole or Molar Pregnancy is a disease of abnormal growth of placental tissue. Most follow a benign course, but a small number of molar pregnancies have the potential for malignancy.
Hydatidiform mole is caused by an aberrant fertilization event.
The result is uncontrolled growth of placental structures called chorionic villi within the uterus.
The structural change is characterized by a mass of vesicles filling and distending the uterus.
The functional change is characterized by the growth of placental tissues from an egg and one or two sperm that have undergone abnormal fertilization without the development of fetal tissues. In a partial mole, a non-viable fetus with multiple anomalies may develop with the placental mass.
The clinical presentation is of abnormal uterine bleeding in the first trimester of pregnancy and a uterus that is larger than expected for a fetus’s gestational age. Imaging is useful in the evaluation of suspected hydatidiform mole with ultrasound. Ultrasound characteristically shows a heterogeneous mass with multiple hypoechoic spaces, described as a “snowstorm” pattern.
Diagnosis is suspected with a combination of clinical presentation and atypical elevation of bhCG, a hormone elevated in pregnancy and a tumor marker for hydatidiform mole.
Treatment for a hydatidiform mole consists of removal of the mass by suction curettage. After removal of a molar pregnancy, levels of bhCG should be monitored for recurrence. In rare cases where an invasive mole develops, a high rate of cure can be achieved with chemotherapy. After removal, subsequent pregnancy and delivery are often possible.
The Common Vein Copyright 2010
Definition
Infertility is a medical condition affecting a couple attempting to conceive. The causes of infertility are variable and may be multifactorial. This condition may be due to either male factors, female factors or both. The result is a couple that is unable to conceive after 12 months of unprotected intercourse. Infertility due to female factors occurs in approximately 50% of cases. Structural changes associated with female-factor infertility include structural disorders of the uterus, cervix and fallopian tubes. Uterine structural abnormalities that cause infertility include congenital uterine and mullerian duct anomalies, uterine polyps and fibroids. Functional changes associated with infertility are often due to ovarian factors with failure of ovulation. However uterine and tubal functional changes like pelvic inflammatory disorder may also contribute to infertility. Couples are diagnosed with infertility after 12 months of unprotected intercourse without conception. However, couples often present clinically before attempting to conceive for 12 months. These couples should be reassured. After 12 months, the diagnosis of infertility is made. Imaging with pelvic ultrasound and hystersalpingography is used to evaluate for female factor infertility. Endocrine and ovulatory factors are also evaluated. Semen analysis is used to evaluate for male factor infertility. Treatment of infertility depends on the underlying cause. For causes that cannot be corrected, ovulation induction, intrauterine insemination and in vitro fertilization can be used to aid a couple in conception.
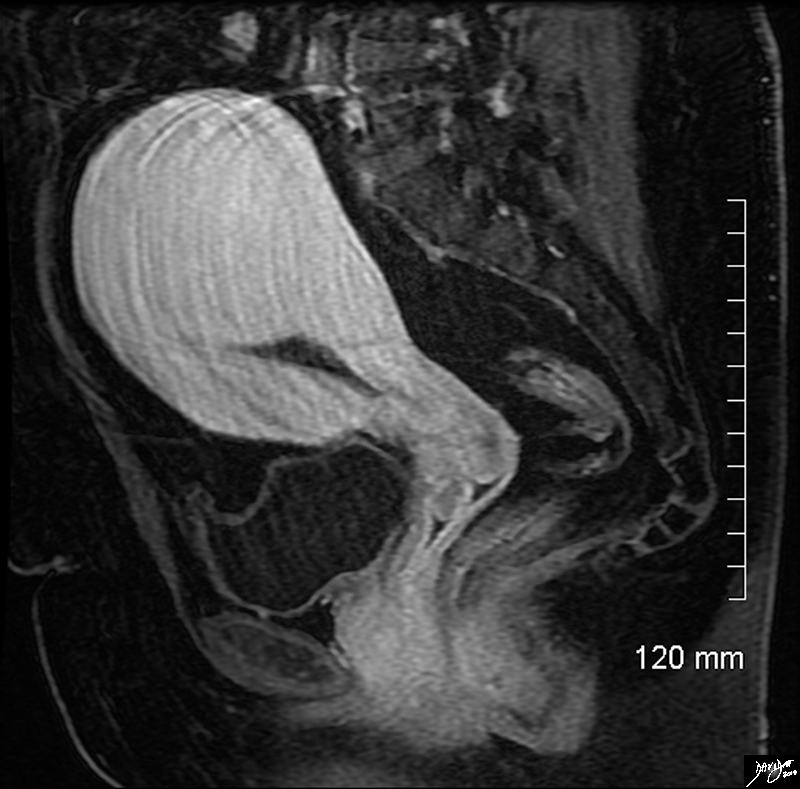
Large Fibroid Impinging on the Emdometrial cavity That Could Resutlt in Infertility |
|
In this sagittal view of the T1 weighted contrast enhanced study of the the uterus of a 46 year old female. The uterus roughly retains its pear shaped structure but the shape of the endometrial cavity is distinctly abnormal as if there is something pushing on it from above. This patient has a diffusely enhancing isointense leiomyoma that impinges on the endometrial cavity. Courtesy Ashley Davidoff MD Copyright 2010 All rights reserved 96577.8s |
|
Two Hemiuteri, Two Cervices and One Vagina MRI T2 Weighted |
|
The MRI is from a 24F year old female with uterus didelphys The T2 weighted study in axial projection reveals 2 uterine corpuses (a) 2 cervices (b), vaginal septum in the upper 1/3 (c) but single distal vagina (d) Image Courtesy Ashley Davidoff MD Copyright 2010 83730c03L.8s |
The Common Vein Copyright 2010
Definiiton
Cervical insufficiency is a structural problem of the cervix that usually occurs late in pregnancy, but can occur as early as the second trimester.
The cause is often a history of cervical surgery or trauma resulting in early dilation of the cervix in pregnancy.
The structural change is characterized by dilation of the cervix before labor with bulging of the fetal membranes through the cervical os.
The functional changes are characterized by an increase risk of early membrane rupture or intrauterine infection.
Women present clinically during routine examination in pregnancy, and are usually asymptomatic.
Diagnosis is made on physical exam. Imaging with ultrasound will sometimes reveal cervical insufficiency in an asymptomatic woman.
Treatment of women with viable pregnancy can be expectant with steroids for fetal lung maturity and strict bed rest. Women with previable pregnancies may be treated with cerclage, a stitch around the cervix to close the os.
The Common Vein Copyright 2010
Definition
Cephalopelvic disproportion is a complication during labor caused by a small or narrow maternal pelvis.
The result is inability of the fetus to descend into the birth canal.
The structural changes are characterized by a narrow maternal pelvis that is too small to allow passage of the fetal head.
The functional changes are characterized by arrest of fetal descent during labor.
Women present clinically with this arrest of fetal descent, termed failure to progress.
Diagnosis is made based on the progression of labor and estimation of maternal pelvic outlet diameter on exam.
Although labor may be attempted in women with suspected cephalopelvic disproportion, treatment of failure to progress is often cesarean delivery.
The Common Vein Copyright 2010
Introduction
The uterus undergoes many functional changes in pregnancy in the processes of implantation, gestation and childbirth.
At the time of ovulation, the endometrium enters its secretory phase. As the sperm move through the uterus, enzymes produced by the endometrial glands catalyze the initial steps of capicitation. Capacitation is an enzymatic reaction that allows sperm to move through the cumulus oophorus and zona pellucida of the ovum. Through their role in capacitation, the endometrial enzymes are necessary components in the process of fertilization. Enzymes from the endometrial glands are also responsible for digestion of the zona pellucida of the fertilized embryo. When the embryo enters the uterus after fertilization, it remains surrounded by the mucopolysaccharide of the zona pellucida. The enzymes form the endometrial glands digest away the mucopolysaccharide, allowing the trophoblastic cells to invade the endometrium and implantation to occur. In the days after implantation, the interstitium and glandular secretions of the endometrium provide nutrition for the developing embryo until the fetal-maternal circulation develops.
During gestation, as the myometrium hypertrophies, it also becomes more contractile. In the first trimester, the uterus begins having infrequent uncoordinated contractions. These contractions are mild and usually go unnoticed. As pregnancy progresses, the contractions increase in intensity and frequency as pregnancy progresses. These contractions become more regular towards the end of the third trimester and become rhythmic as labor begins.
As labor begins, there is an increase in the number of oxytocin receptors on the myometrial smooth muscle cells. Release of the hormone oxytocin from the posterior pituitary stimulates the strong rhythmic contractions of the myometrium. There is also an increase in the number of gap junctions connecting the smooth muscle cells. As a result, the contraction of the myometrial smooth muscle cells becomes more coordinated. In the cervix, increased prostaglandin levels lead to increased expression of enzymes called matrix metalloproteinases. These enzymes break down cross-linking bonds between collagen fibers. Simultaneously, cervical hyaluronic acid content increases. These processes cause the softening, effacement and dilation of the cervix. Once the cervix is dilated, rhythmic myometrial contractions move the fetus through the birth canal. The ligaments of the pelvis relax to allow widening of the pelvic outlet, allowing the fetus to pass. After the delivery of the neonate and separation of the placenta, constriction of the uterine vasculature prevents maternal blood loss. Contraction of the
The Common Vein Copyright 2010
Introduction
Uterine structural changes are necessary to allow implantation, gestation and childbirth.
Early in pregnancy, the endometrium provides a suitable environment for implantation of the embryo. The abundant glands and stroma formed in the proliferative phase of the menstrual cycle provide a site for trophoblastic invasion. In the secretory phase these glands will also produce several products necessary for fertilization and implantation. The endometrial vascular proliferation of the secretory phase provides a rich blood supply for the embryo after implantation.
After implantation the muscle cells of the myometrium hypertrophy. This leads to an initial increase in the thickness of the uterine wall. However as the fetus grows, the uterine wall stretches and thins. The elastic and fibrous tissue content of the myometrium also increases. This allows the uterus to stretch and accommodate the growing fetus, placenta and amniotic fluid. By the end of pregnancy the uterus has increased in size 500 times. Vascular growth and vasodilation of the uterine vasculature increases blood flow to the uterus as the pregnancy progresses to provide fetal nourishment through the maternal-fetal circulation.
During labor the cervix undergoes important structural changes to allow delivery. Prostaglandins lead to increased enzymatic activity dissolving the cross-linking bonds between collagen fibers. This results in increased cervical elasticity, cervical effacement and cervical dilation. The pelvic outlet widens to allow the fetus to pass, a result of pelvic ligament stretching and relaxation. As the fetus passes into the birth canal, the uterus maintains contraction, transforming from a thin-walled organ with a large central cavity to a thick muscular organ with almost no space in the central cavity. During delivery, the muscles of the pelvic floor thin allowing the vaginal opening to stretch during childbirth. After delivery, constriction of the uterine vasculature decreases maternal blood loss.
|
Junctional Zone In Early Proliferative Phase |
|
The normal sagittal view of the uterus is a transvaginal ultrasound, in the first week after menstruation after menstruation, and just prior to the next menstruation after the endometrium has been shed. It demonstrates that the endometrium becomes a single echogenic line consisting of opposing walls (orange) and is surrounded by a subendometrial halo of the junctional zone (tan). This layer is more compacted, and relatively hypovascular. This image is typical of the early proliferative phase. It is during this time that estrogen starts to rise and progesterone has fallen. The endometrium in this case measures about 3mms. Courtesy Ashley Davidoff MD Copyright 2010 All rights reserved 84698c02b.8s |
|
Junctional Zone in the Premenstrual Uterus LMP 4 Weeks Prior |
|
In this 26 year premenstrual female a transvaginal ultrasound in the sagittal plane reveals a normal view of the uterus with characteristic premenstrual appearance. (a) The stripe is almost homogeneously echogenic and thick but also shows a hypoechoic halo of the junctional zone or inner myometrium. (salmon) The homogeneous stripe is made up from two histological layers (barely distinguished by this ultrasound)– the inner stratum functionalis (deep orange) that will shed once the spiral arteries vasoconstrict, and the outer stratum basalis (deep yellow) that will not shed, and will be the basis for regenerating the endometrium in the next cycle. The next layer as stated above is the compact myometrium – the junctional zone (aka inner myometrium) , and is followed by the thicker outer myometrium (maroon). (b) Courtesy Ashley Davidoff MD Copyright 2010 All rights reserved 84538c06.83s |
Junctional Zone During Pregnancy
The junctional zone becomes disrupted increases in intensity and zonal differences become indistinct and returns to normal 6 months after deliver Willms AB Brown ED Keittritz UIRadiology 195 91-94 1995
|
Junctional Zone – Early Pregnancy |
|
In this 16 year old patient her LMP was about 4 weeks ago and she had a positive pregnancy test. The transvaginal ultrasound in the sagittal plane reveals a normal view of the uterus with characteristic early pregnancy appearance characterized by the gestational sac (gs) embedded in the stratum functionalis (deep orange). The stratum basalis is seen as a slightly more echogenic layer around the functional layer (deep yellow). The stripe is thick measuring about The next layer is a barely seen junctional layer (salmon pink) best seen on the anterior subendometrial layer just beyond the basalis The next layer is the thicker outer myometrium (maroon) that contains dilated vessels. Courtesy Ashley Davidoff MD Copyright 2010 All rights reserved 84485c02.8ls |
|
|
Early in pregnancy, the endometrium provides a suitable environment for implantation of the embryo. The abundant glands and stroma formed in the proliferative phase of the menstrual cycle provide a site for trophoblastic invasion. In the secretory phase these glands will also produce several products necessary for fertilization and implantation. The endometrial vascular proliferation of the secretory phase provides a rich blood supply for the embryo after implantation.
After implantation the muscle cells of the myometrium hypertrophy. This leads to an initial increase in the thickness of the uterine wall. However as the fetus grows, the uterine wall stretches and thins. The elastic and fibrous tissue content of the myometrium also increases. This allows the uterus to stretch and accommodate the growing fetus, placenta and amniotic fluid. By the end of pregnancy the uterus has increased in size 500 times. Vascular growth and vasodilation of the uterine vasculature increases blood flow to the uterus as the pregnancy progresses to provide fetal nourishment through the maternal-fetal circulation.
During labor the cervix undergoes important structural changes to allow delivery. Prostaglandins lead to increased enzymatic activity dissolving the cross-linking bonds between collagen fibers. This results in increased cervical elasticity, cervical effacement and cervical dilation, allowing childbirth. After delivery, constriction of the uterine vasculature decreases maternal blood loss.
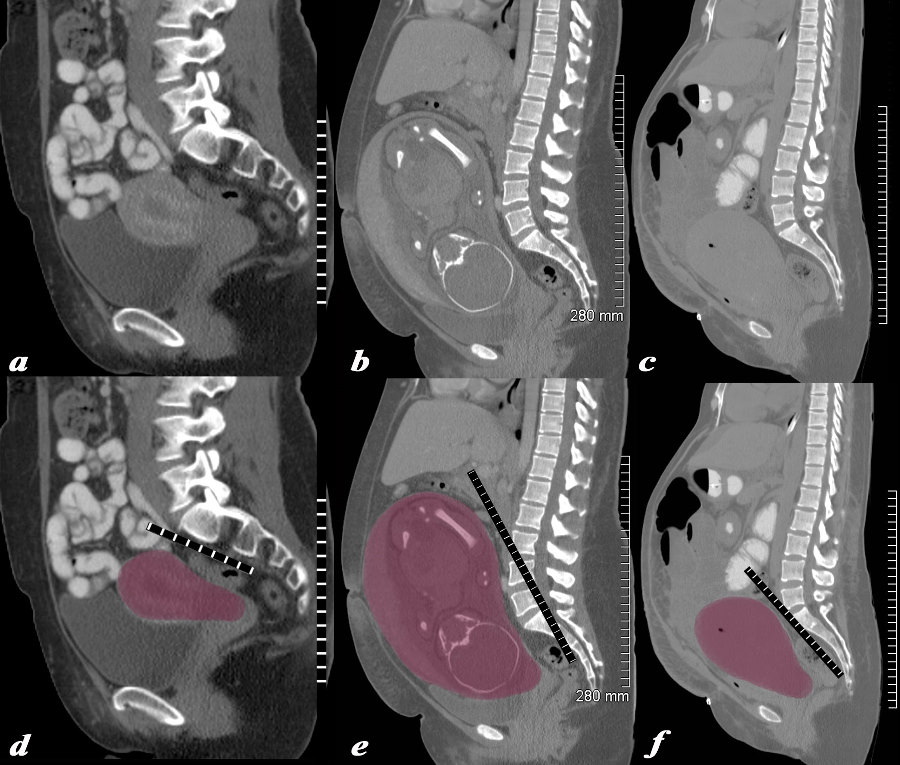
Structural Changes Non Gravid, 32 Week Pregnancy, and Post Cesarean Section |
|
The series of CT scans from different patients are reconstructed in the sagittal plane to show the mature uterus in the non-gravid state (a,d) with a 32 week pregnancy (b,e), and in the postpartum, post cesarean section state (c,f). In the nongravid adult the uterus the craniocaudad span (c-c) measures about 9cms and the anteroposterior (A-P) dimension it measures 4.5cms The uterus containing the 32week pregnancy measures 24cms (c-c) by 16cms (A-P). In the post cesarean section patient the uterus measures 17cms (c-c) by 9cms (A-P). Courtesy Ashley Davidoff MD Copyright 2010 All rights reserved 78093c08.8s |
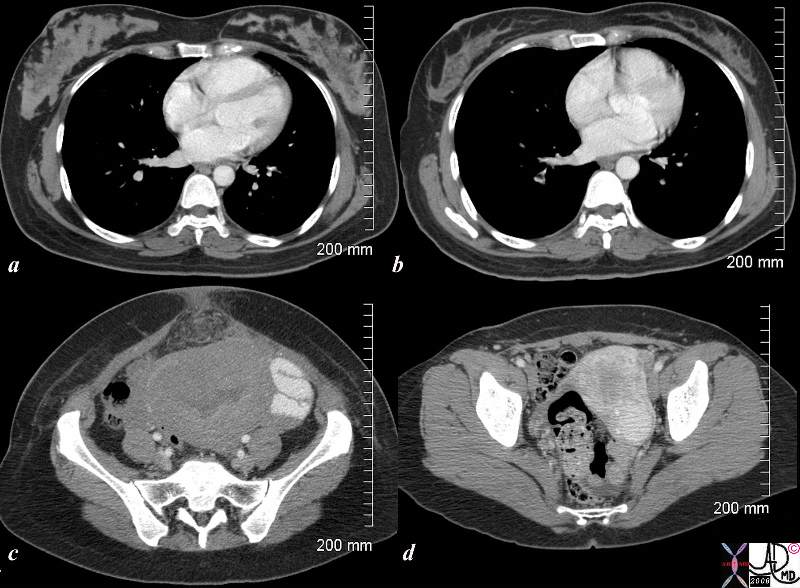
Postpartum and 18months Later |
|
The CTscan is from a 26 year old female showing breasts and uterus in a post partum post cesarian section state (a,c) and then 18months later (c,d). The transverse dimension of the uterus in the post partum state is about 11cms, while 18months later is about 5cms. Her breasts in the post partum state are enlarged with prominent glandular tissue (a), and in the post partum state are reduced in size and glandular volume (b). Surgical footprints are noted in the subcutaneous tissue in c following her cesarian section. A cervical fibroid is suggested post partum image (d). Courtesy Ashley Davidoff copyright 2009 83354c.8s |